
/atomic-structure-680789951-5919e8e83df78cf5fa739b46.jpg)
It is composed of protons, which have a positive charge, and neutrons, which have no charge. The nucleus is the positively charged centre of an atom and contains most of its mass. Attempts to separate these smaller constituent particles require ever-increasing amounts of energy and result in the creation of new subatomic particles, many of which are charged.Īs noted in the introduction to this article, an atom consists largely of empty space. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Each individual atom consists of smaller particles-namely, electrons and nuclei. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. For additional information pertaining to nuclear structure and elementary particles, see subatomic particles.ĭo you get fired up about physics? Giddy about geology? Sort out science fact from fiction with these questions. Following this overview is a historical survey of the most influential concepts about the atom that have been formulated through the centuries. This article opens with a broad overview of the fundamental properties of the atom and its constituent particles and forces.
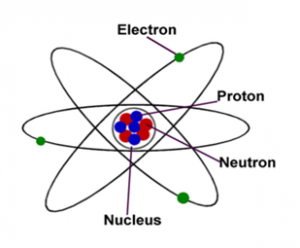
The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells. Such wave patterns, called orbitals, describe the distribution of individual electrons. In others, the electrons behave like waves frozen in position around the nucleus. In some respects, the electrons in an atom behave like particles orbiting the nucleus. Electrons are attracted to any positive charge by their electric force in an atom, electric forces bind the electrons to the nucleus.īecause of the nature of quantum mechanics, no single image has been entirely satisfactory at visualizing the atom’s various characteristics, which thus forces physicists to use complementary pictures of the atom to explain different properties. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. The rest consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Investigate varying electron configurations in electron shells around an atom's nucleus See all videos for this article SpaceNext50 Britannica presents SpaceNext50, From the race to the Moon to space stewardship, we explore a wide range of subjects that feed our curiosity about space!.Learn about the major environmental problems facing our planet and what can be done about them! Saving Earth Britannica Presents Earth’s To-Do List for the 21st Century.Britannica Beyond We’ve created a new place where questions are at the center of learning.100 Women Britannica celebrates the centennial of the Nineteenth Amendment, highlighting suffragists and history-making politicians.

ATOM EXAMPLES HOW TO


 0 kommentar(er)
0 kommentar(er)
